Let’s Talk about Hydrogels
How PVA-Based Materials are Transforming Soft Tissue Simulation in Medical Training
Introduction
Hydrogels are having a moment in the medical world - but not only in futuristic research labs where scientists are printing heart valves or dreaming of organ regeneration. In the here and now, hydrogels are quietly transforming how healthcare professionals train, plan, and prepare for complex interventions.
At HumanX Medical, we work with hydrogels for a very different (but incredibly practical) purpose: to replicate soft human tissue for use in surgical training, skills development, and product testing. Our models aren’t grown in bioreactors or seeded with living cells. They’re built for realism, durability, and repeatability. And they’re already in use in skills labs, research departments, and simulation centers worldwide.
This article focuses specifically on PVA-based (polyvinyl alcohol) hydrogels, a family of versatile, non-toxic, water-rich materials that can be tailored to mimic everything from muscle and liver to the delicate walls of the uterus or appendix. These materials are not designed to replace real organs in the body, but to replace animals and cadavers in the training room.
We’ll walk through what makes hydrogels so powerful in simulation, how they’re made, how their properties can be customized, and where the field is heading next. You’ll also get a look into our own journey: from our first liver model developed in collaboration with a German Research Institution to the creation of the modular EasySurg Training System that now supports a growing line of SimOrgan hydrogel replicas.
Whether you’re a surgeon-in-training, a medical device designer, or an educator looking for better tools, this article will give you an inside look at one of the most promising and practical materials shaping the future of medical simulation.
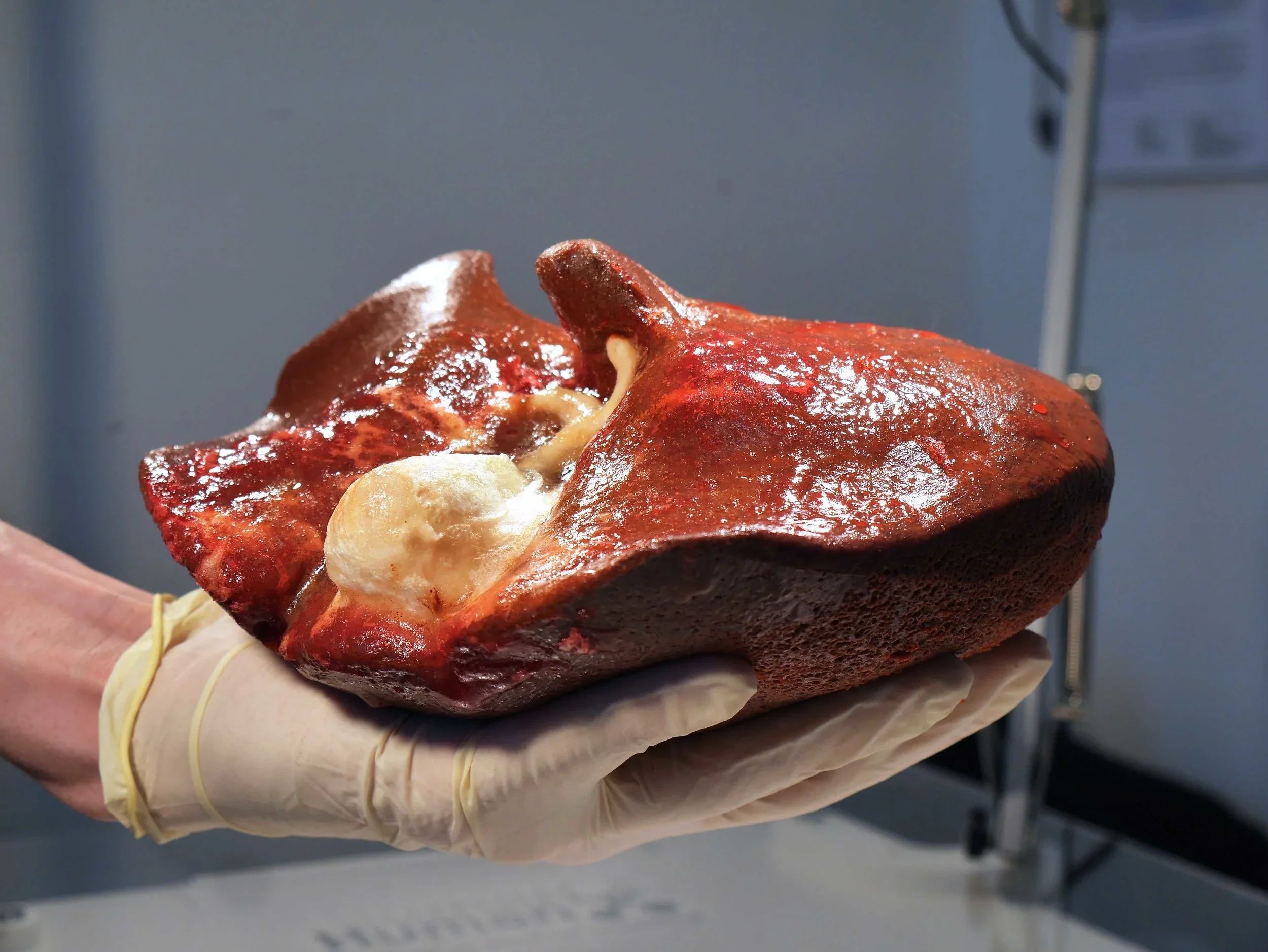
Hydrogel-based model of a Liver
Source: HumanX Medical
1. Why Hydrogels Matter in Medical Simulation
Ask any surgeon, interventionalist, or device engineer what’s missing from most simulation models, and the answer is almost always the same: realism. It’s one thing to understand anatomy from a textbook or even a 3D-printed plastic replica - but it’s another entirely to insert a needle, apply surgical force, or feel resistance while navigating tissue layers that behave like the real thing.
In training environments, we’re often stuck with two extremes:
On one side, hard plastics and silicone models that are durable but lifeless, offering no realistic feedback.
On the other, biological specimens - human cadavers or animal tissues - that may be anatomically correct, but are costly, perishable, ethically complicated, and nearly impossible to standardize (See our last article “Beyond meat”).
This is where hydrogels come in.
Hydrogels - particularly those made from polyvinyl alcohol (PVA) - can hold over 90% water while remaining structurally stable. This makes them uniquely suited to replicate soft, hydrated tissues like liver, muscle, brain, and even pathological structures like tumors or cysts. Their tactile behavior under compression, tension, or puncture is remarkably close to living tissue - without the ethical baggage or logistical overhead.
What’s more, hydrogel models can be cast into anatomically accurate forms using STL files derived from medical imaging, allowing for high-fidelity training on both normal and pathological anatomy. They can even be adapted to respond to ultrasound or fluoroscopic imaging by incorporating radiopaque or echogenic additives.
In short, hydrogels fill a long-standing gap in simulation:
They are soft, but structured
Realistic, but repeatable
Accessible, yet advanced
And they do all this while supporting broader goals in medical education and R&D - such as improving procedural confidence, reducing reliance on live animals, and enabling safer, more efficient testing environments.
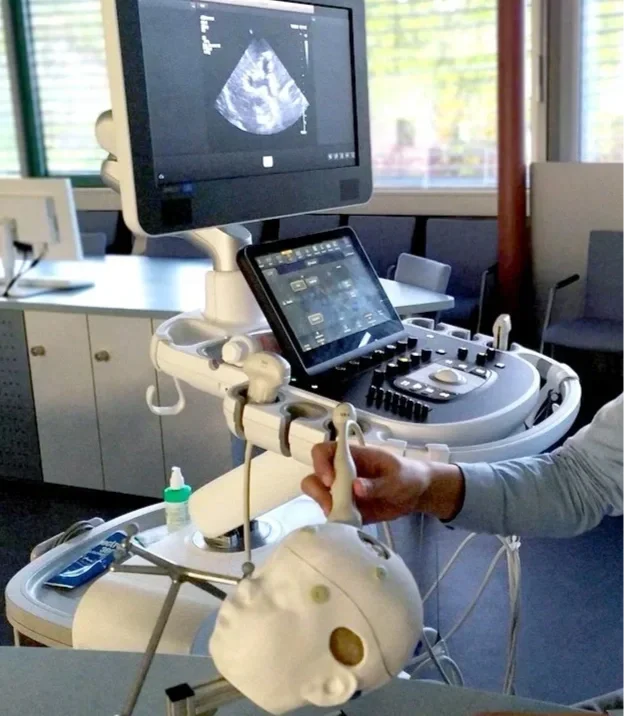
Ultrasound training for pediatric neurosurgery using a hydrogel-based training model (NeuroLisa by HumanX).
Source: HumanX Medical
2. What Is PVA – And Why It Works So Well
At the heart of most high-performance hydrogel models used in medical simulation is a material called polyvinyl alcohol, or PVA. It’s not new. In fact, PVA has been around for decades—used in everything from contact lenses to biodegradable packaging. But in the right formulation, and with the right curing process, PVA becomes something far more interesting: a soft, resilient, tissue-like medium that holds its shape, tolerates instrumentation, and delivers a remarkably life-like feel.
So, what makes PVA such a good fit for soft tissue replication?
Biocompatibility: PVA is non-toxic and widely regarded as safe for skin contact and surgical training environments. That means no need for complex handling protocols or hazardous chemical disposal.
Water Affinity: Once hydrated and crosslinked, PVA can hold up to 90% water, closely mimicking the hydration level of human organs. This water-rich environment is key to getting the right softness, texture, and responsiveness under pressure.
Tunable Mechanics: By adjusting the concentration of the polymer and the curing method (more on that in the next section), PVA hydrogels can be made softer than fat or firmer than muscle. This offers a wide range of applications from fine-needle puncture to deep-cutting procedures.
Imaging Compatibility: With the right additives PVA hydrogels become visible on medical imaging, making them ideal for procedural navigation or device tracking.
Compared to other hydrogels like agarose or PEG (polyethylene glycol), PVA stands out for its mechanical durability. Some hydrogels tear easily or degrade after a single use. But a properly cured PVA model can withstand multiple cuts, sutures, or injections, making it a cost-effective option for repeated training sessions.
At HumanX Medical, we’ve worked extensively with PVA to match not just the appearance - but the tactile behavior - of specific human organs. Whether simulating the soft, yielding nature of a healthy organ or the denser feel of a fibrotic tissue, PVA gives us the range and control we need to deliver accurate, high-fidelity models for a variety of applications.
3. Multiple Ways to Prepare and Cure PVA Hydrogels
One of the key strengths of PVA-based hydrogels is their flexibility in production. Depending on the desired properties, resources available, and application, there are several methods to transform liquid PVA into a solid, tissue-like hydrogel. Each curing process creates a different internal structure - affecting softness, elasticity, and durability.
Let’s look at the three most common methods used in hydrogel fabrication today:
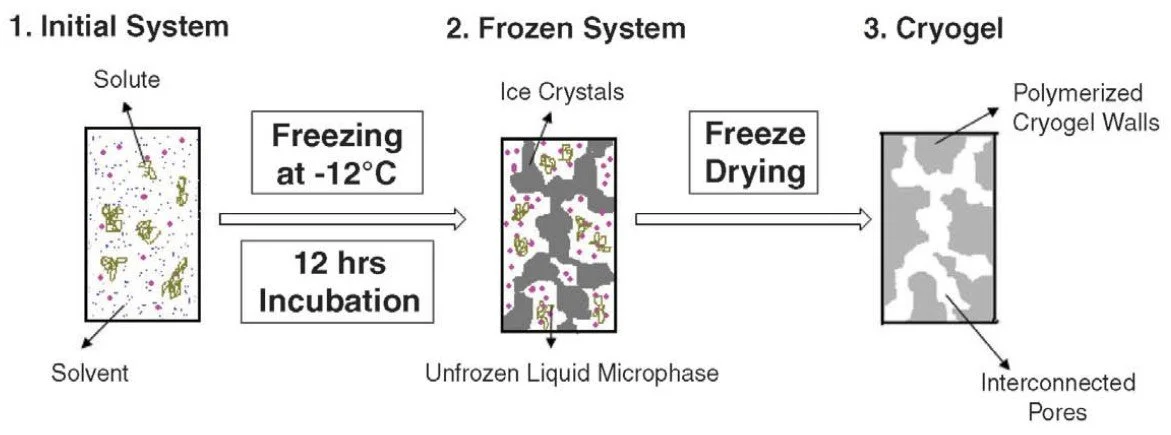
3.1. Freeze–Thaw Cycles (Cryogelation)
This is the most widely used and safest method, especially for surgical simulation models. The process involves repeatedly freezing and thawing a PVA solution. During freezing, water forms ice crystals that push the polymer chains together. Upon thawing, these chains remain physically bonded, forming a stable and flexible gel.
Advantages: No chemical crosslinkers, excellent biocompatibility, easy to scale
Adjustability: The more freeze–thaw cycles applied, the stiffer the final hydrogel becomes
Best suited for: Hands-on surgical training, needle-based procedures, anatomical modeling
At HumanX Medical, this is our go-to technique for SimOrgan models, delivering realism with no toxic byproducts.
Source: Materials Today, Volume 13, Issue 11, November 2010, Pages 42-44
3.2. Chemical Crosslinking
In this method, chemical agents like glutaraldehyde or boric acid are added to initiate crosslinking between PVA molecules.
Advantages: Faster curing time, ability to fine-tune mechanical properties and swelling behavior
Challenges: Requires careful handling due to potential toxicity of residual chemicals
Best suited for: Lab-controlled environments, research purposes, or prototypes with enhanced stiffness
While not ideal for all training environments, chemical crosslinking can be useful when more control over gel architecture is needed or when hybrid material systems are being explored.
3.3. Radiation Crosslinking (Gamma, UV, or Electron Beam)
A less common but highly precise method, radiation crosslinking exposes the PVA solution to controlled energy sources, which initiate bonding between polymer chains without chemical additives.
Advantages: Clean, sterile, and tunable down to the microstructure
Challenges: Requires specialized equipment, strict safety protocols, and often higher cost
Best suited for: Research institutions, advanced imaging phantoms, or high-end custom simulations
Each of these methods creates a slightly different outcome, and the choice depends on the balance between performance, cost, safety, and complexity. For most simulation environments, freeze–thaw cryogels offer the best tradeoff between realism, reproducibility, and ease of preparation.
In fact, the simplicity of the freeze–thaw method is what allows HumanX customers and partners to produce their own hydrogel models in-house using our SimOrgan DIY guide without the need for hazardous substances or expensive lab setups.
4. From Recipe to Realism – Tuning Material Properties
One of the greatest advantages of working with PVA hydrogels is how precisely they can be tuned to replicate the feel of different human tissues. By adjusting a few key variables in the preparation process, it's possible to create models that simulate the delicate, almost gelatinous texture of a fatty liver - or the dense resistance of a muscular organ under tension.
Here’s how that level of realism is achieved:
4.1. Polymer Concentration
The starting concentration of PVA in water determines the base stiffness of the hydrogel.
Lower concentrations (4–6%) yield very soft, flexible gels suitable for mimicking tissues like brain, fat, or fluid-filled cysts.
Higher concentrations (10–15%) create firmer, more elastic structures like muscle, uterus, or dense tumors.
4.2. Number of Freeze–Thaw Cycles
Each cycle increases the degree of physical crosslinking, resulting in a stronger and more structured gel.
Fewer cycles = softer, more pliable material
More cycles = greater resistance to cutting, stretching, and suturing
This adjustability is what allows HumanX to offer SimOrgan models in different grades, from ultrasoft to firm, depending on the training objective.
4.3. Additives and Modifiers
The behavior and function of the hydrogel can be enhanced by adding specific agents:
Plasticizers like glycerol increase flexibility and reduce drying
Nanoparticles or bioceramics can replicate tissue resistance or add opacity
Colorants allow for tissue differentiation, pathology simulation, or surface visualization
Ionic Compounds like NaCl create an electrolytic solution when dissolved in the hydrogel to allow mono- and bipolar HF-surgery
These modifications turn a simple gel into a multifunctional training tool, supporting not only tactile realism but also imaging-based procedures.
4.4. Layering and Hybrid Structures
Advanced training models often require simulating more than one type of tissue in a single structure - for example, a uterus with a tumor mass, or a vascular structure embedded in soft tissue.
To meet these demands, hydrogels can be cast in layers, using different recipes for each layer or region. This enables the creation of:
Multi-density phantoms
Tumor-mimicking inclusions
Vessels, ducts, or voids within solid tissue
This level of control is what makes PVA hydrogels such a powerful material for modern medical simulation. It’s not a one-size-fits-all approach - it’s a recipe-driven system that can be adapted to match the mechanical and visual requirements of nearly any procedure.
5. 3D-Printable Hydrogels – A Glimpse into the Future
Imagine being able to print soft tissue models on demand - with complex internal structures and precise geometries - all tailored to a specific training scenario. That vision is gradually becoming reality, thanks to evolving 3D-printing methods.
However, not all 3D printing is created equal, especially when it comes to PVA-based hydrogels. Fused Deposition Modeling (FDM), prevalent in rigid plastics, doesn’t suit PVA hydrogels due to its thermal melting process and structural limitations. Instead, research points clearly toward photopolymerization-based techniques like SLA and DLP for achieving high-fidelity, soft hydrogel parts.
Why SLA and DLP Work Better for Hydrogels
Stereolithography (SLA) uses a UV laser to selectively cure photosensitive resins, layer by layer, forming detailed 3D structures. This precision is ideal for intricate soft-tissue models.
Digital Light Processing (DLP) cures entire layers at once using projected light. It's faster than SLA, and resolution can reach down to tens of microns, making it particularly suitable for soft, flexible structures like hydrogels.
Studies highlight that both methods excel in producing high-resolution, flexible, and complex geometries - qualities essential for simulating nuanced anatomical features or pathological variations.
Innovations in 3D‑Printable PVA Hydrogels
While traditional PVA isn’t inherently UV-curable, recent research has shown promising developments:
Intrinsic photo-curable PVA variants are enabling high-resolution DLP printing of realistic, biocompatible hydrogel models.
Another approach demonstrated the use of an aqueous two-phase system (ATPS), enabling Direct Ink Write (DIW) printing of PVA hydrogels in moderate alkaline conditions. This technique stabilizes printed layers long enough for gradual physical crosslinking.
While these innovations are still emerging and not yet ready for every lab or simulation kit, they point toward a future where PVA hydrogels can be 3D printed with precision, repeatability, and structural integrity.
What This Means for Medical Simulation
Customization: Ability to print organ-specific models with patient-based geometries or rare pathological features using DLP/SLA.
Speed & Resolution: Faster printing cycles and high-detail resolution make DLP especially promising for rapid prototyping.
Current Constraints: These methods require photosensitive PVA formulations and controlled settings - making them better suited for labs with advanced tech setups.
At HumanX, we’re working on these developments. While our current methods rely on traditional, reliable molding techniques like freeze–thaw cryogels, the potential of photo-curable PVA hydrogels is exciting. We anticipate future workflows that blend the robustness of cast hydrogels with the flexibility of cutting-edge printing - a hybrid approach that could offer both realism and agility.
6. Best Practices for Hydrogel-Based SimOrgan Models
As with any material used in medical training and simulation, hydrogel models deliver the best results when prepared, stored, and handled correctly. Over the past several years, we’ve refined a number of best practices that help ensure durability, realism, and repeatable performance, especially for our PVA-based SimOrgan models.
Whether you're producing these models yourself using our how-to manual or working with pre-fabricated units, the following principles can make a significant difference in quality and longevity:
6.1. Hydration Is Key
PVA hydrogels are water-rich materials, holding up to 90% water depending on the formulation. To maintain their softness and elasticity:
Always store models in a sealed container, preferably in distilled water or saline
For longer-term storage, keep models refrigerated (4–8 °C) to slow dehydration and microbial growth
If a model begins to dry out, gently rehydrate it over several hours (avoid soaking in hot or chlorinated water)
6.2. Know the Limits of Reusability
Hydrogel models can tolerate multiple procedures such as:
Needle puncture
Suturing
Cutting
Injection
But their lifespan depends on the intensity and type of use. For example:
A soft liver model used for injection training may last for 15+ sessions
A uterus model used for surgical dissection may show wear after the first run
If a model becomes torn or structurally unstable, it’s best to retire and recast it.
6.3. Clean and Disinfect Carefully
Hydrogels are non-toxic but can become contaminated through repeated use. To maintain hygiene:
Rinse with isotonic saline or distilled water after each use
Use non-alcoholic disinfectants only. Alcohols or strong oxidizers can damage the matrix
Avoid autoclaving or dry heat sterilization (which will dehydrate or melt the hydrogel)
6.4. Color Coding and Layering
For visual realism and training clarity, many SimOrgan models include:
Color differentiation between tissue types (e.g., muscle vs. tumor)
Multilayer structures, with varying softness and texture
Surface coatings (optional) to improve traction or visualization during handling
When casting your own models, using simple food-grade dyes or layering different hydrogel formulations can greatly improve the educational value of the final model.
6.5. STL Files and Mold Design
Hydrogels can be cast into almost any shape, especially when based on real medical imaging data (CT or MRI).
At HumanX, we provide STL files for a range of anatomical structures, which can be used to 3D print molds
Models created from DICOM-to-STL pipelines allow for patient-specific simulation and pathology replication
If you’re developing custom models, remember:
Smooth surfaces help reduce tearing
Undercuts can complicate demolding
Split-mold designs ease the removal of soft models after casting
Proper care and design can extend the lifespan of your hydrogel models, enhance the fidelity of your training programs, and reduce the total cost of ownership compared to single-use specimens or cadaveric tissue.
7. The HumanX SimOrgan Journey – From Soft Tissue to Simulation Systems
At HumanX Medical (and former German-based HumanX GmbH), our work with hydrogels isn’t theoretical, it’s rooted in years of practical development, driven by the need for more realistic, ethical, and versatile simulation tools in healthcare training. The journey began in 2017, when we launched our first major hydrogel project in partnership with FILK Freiberg gGmbH, a German research institute with deep expertise in functional polymer materials.
The collaboration focused on a deceptively simple challenge:
Could we replicate the look and feel of a human liver, soft, lifelike, and instrument-ready—without relying on cadaver tissue?
The result was our first SimOrgan hydrogel liver model, a highly realistic phantom that offered not only the tactile behavior of human liver tissue, but also the ability to be punctured, injected, dissected, and imaged. When we presented the model to leading medical device manufacturers and university partners, the feedback was overwhelmingly positive: finally, a training tool that balanced anatomical accuracy with practical usability.
Expanding the Product Line
Encouraged by the success of the liver model, we expanded the SimOrgan line to include:
Appendix models for endoscopic training
Uterus phantoms for surgical and procedural rehearsal
Prostate replicas for device demonstration and urology training
Each model was designed with specific procedural goals in mind: from practicing injections or resections to testing new device approaches under ultrasound or X-ray guidance.
Bringing Simulation to Life with EasySurg
By 2019, it became clear that the hydrogel models needed more than just anatomical accuracy: they needed a realistic environment that could replicate patient positioning, access constraints, and procedural ergonomics.
That led to the development of the EasySurg Training System:
A modular platform designed to house SimOrgan models in surgical-like conditions, complete with mounting options, camera access, and interchangeable anatomical inserts.
This “stage” allowed our soft-tissue phantoms to perform in high-fidelity simulations, whether in a skills lab, training workshop, or device demonstration booth.
From its origins in material research to its application in complex simulation scenarios, the SimOrgan hydrogel line reflects our belief that medical training deserves more than rigid plastics or expired specimens. It deserves tools that feel real, respond naturally, and can be tailored to the specific challenges of modern procedures.
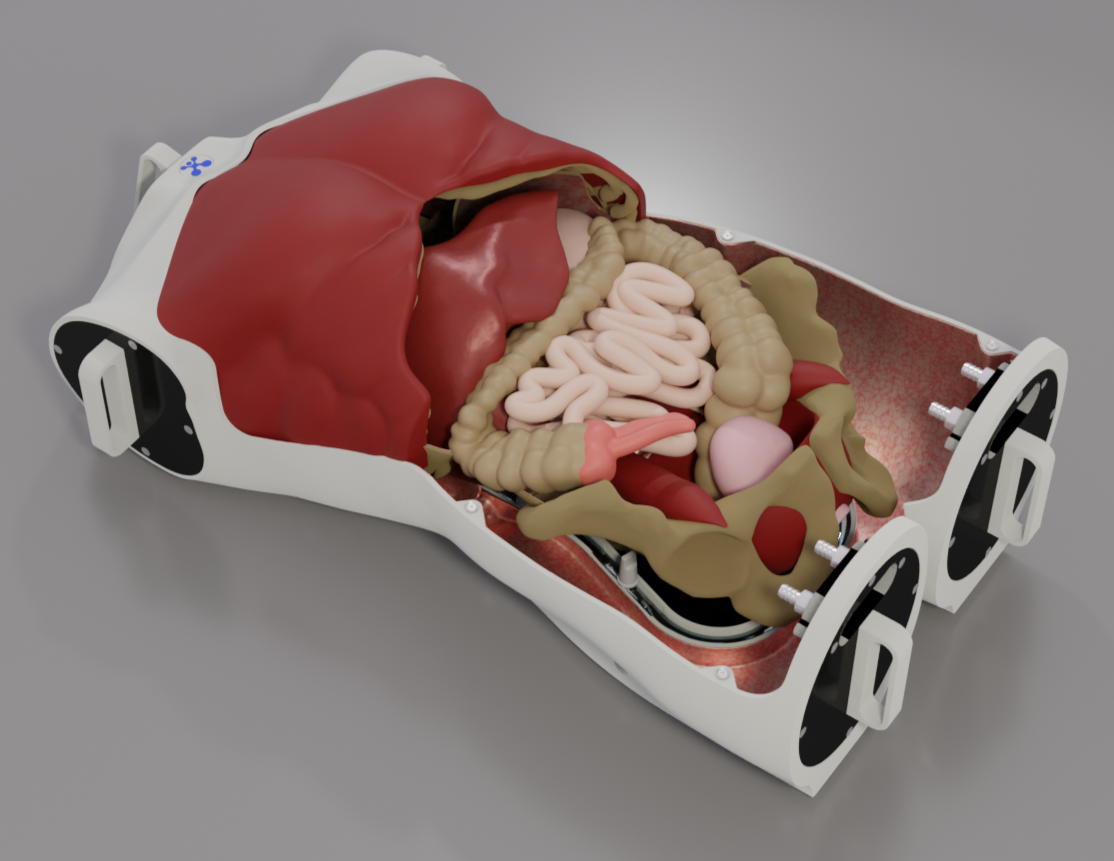
Perfect Environment for hydrogel-based organ models: EasySurg Training System
Source: HumanX Medical
8. Conclusion: Hydrogels as a Bridge Between Ethics and Realism
In today’s evolving landscape of medical training and simulation, the search for materials that balance realism, scalability, and ethical responsibility is more important than ever. PVA-based hydrogels stand out as one of the few materials capable of bridging that gap, providing life-like tactile feedback without relying on human cadavers or animal specimens.
Through years of hands-on development, HumanX Medical has demonstrated that these materials are more than a scientific curiosity. Hydrogels have become a practical, proven, and production-ready solution for replicating soft tissue anatomy, used today in procedure training, device demonstrations, and clinical dry-runs around the world.
They support the 3R principles (Reduce, Replace, Refine) in a tangible way, helping institutions move toward simulation-based alternatives that are more sustainable, more reproducible, and ultimately more effective.
And as research continues to expand into 3D-printable hydrogels, UV-curable variants and hybrid manufacturing workflows, the opportunities to customize, personalize, and scale soft tissue simulation are only growing.
Whether you're a clinical educator, a medical device innovator, or a research leader, hydrogels represent a toolkit worth exploring, not because they're futuristic, but because they're ready now.
We invite you to join us in rethinking how simulation can serve as both a scientific and ethical evolution in medical training.
Disclaimer: About the Use of Hydrogels in Medical Simulation
The hydrogel-based models described in this article are intended solely for educational, training, and device demonstration purposes. They are non-living, non-biological simulators and are not designed or intended for implantation, therapeutic use, or regenerative medicine.
While hydrogels are frequently referenced in the context of bioprinting or tissue engineering, the materials and methods discussed here - particularly PVA-based cryogels and UV-crosslinkable formulations - are not suitable for organ replacement or transplant research.
At HumanX Medical, our focus lies in the development of high-fidelity anatomical replicas to support realistic, ethical, and reproducible medical simulation environments. These tools serve as practical alternatives to cadaveric or animal specimens and help fulfill the principles of Reduce, Replace, and Refine (3R) in modern healthcare education.